|
|
|
LC Method Validation™ - Automated Method Validation Software
|
|
|
Works with Waters® and Agilent LC Systems controlled by:
|
- Waters Empower™ (1 and 2) Chromatography Data Software (CDS)
- Agilent Chemstation® and Galaxie® CDS
- Dionex® Chromeleon® Chromatography Data Software (CDS)
|
|
|
|
PhRMA’s Analytical Technical Group recommends a phased approach to analytical method validation in which early phase validation efforts are done upstream on a reduced set of validation elements appropriate to the stage of development.
|
|
|
|
Early Phase Method Validation (Characterization)
|
|
|
Fusion Method Validation™ (FMV) offers Early Phase experiments structured for internal consumption to support and guide method development.
- Analytical Capability and System Suitability
- Specificity
- Filter Validation
- Accuracy
- Linearity and Range
- LOQ, LOD
- Repeatability* (intra-assay precision)
- Sample Solution Stability (stability for a given time period under prescribed conditions)
|
|
|
|
Final Phase Method Validation (FDA and ICH Submittal Quality)
|
|
|
FMV offers Final Phase experiments structured with the rigor and regulatory compliance overlay required of results that may be exported outside the lab.
- Analytical Capability and System Suitability
- Specificity
- Accuracy/Linearity and Range/Repeatability – Combined Design (ICH-Q2A states that Accuracy, Linearity, and Repeatability can be done together as a single combined experiment)
- LOQ, LOD
- Intermediate Precision and Reproducibility (USP Ruggedness)
- Robustness
|
|
Pharma Customer Benchmarking
|
|
|
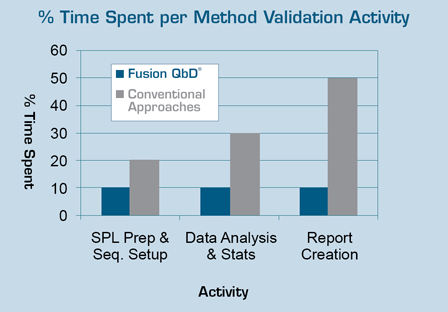
To benchmark time savings using FMV versus current practice, a senior analytical chemist at an international pharmaceutical account used FMV to complete a series of Early Phase and Final Phase method validation experiments. The entire method validation exercise took less than 12 hours using Fusion AE.
Work records showed that on average - from SOP planning and experiment design construction to final reporting - using “current practice” this project would have required more than two weeks of analyst time.
|
|
|
|
|
|
|
|
For more information on Fusion LC Method Validation, contact us at 707-441-0404 or sales@smatrix.com
|

