|
|
|
LC Method Development™ - Automated Method Development Software
|
|
Works with all Waters® and Agilent LC Systems controlled by the Waters Empower™ (1 and 2) Chromatography Data Software
|
|
|
|
Turn Your LC into an Automated QbD Method Development System
|
|
|
Fusion LC Method Development™ (FMD) controls most internal and external column switching valves and solvent selection valves for your Waters or Agilent LC system. This enables you to do rapid method development for all types of LC chromatography, including Reversed Phase, Normal Phase, Chiral, HILIC, Ion Exchange, and Size Exclusion. You can use FMDs standard screening and optimization experiment templates or create your own.
|
|
|
|
Phase 1 - Rapid Column and Solvent System Screening
|
|
|
FMD brings a new approach to automated LC column and solvent system selection that is completely aligned with the principles of QbD. S-Matrix’s patented Trend Responses™ technology (U.S. Patent No. 7,613,574 B2) overcomes the limitations inherent in both the sequential and classical Design of Experiments (DOE) approaches and places column and solvent screening method development activities on a rigorous and quantitative footing. Most importantly, the Trend Responses approach eliminates the requirement for laborious and error-prone peak tracking in phase 1 column and solvent system screening experiments.
|
|
|
|
Phase 2 - Robust Methdod Development and Optimization
|
|
|
FMD brings a new QbD-based methodology to formal HPLC method development. Regulatory guidances state that the best-practices approach should address robustness during formal method development. Therefore, a critical feature of FMD is our patented Robustness Simulator™ technology (U.S. Patent No. 7,606,685 B2), which integrates automatically computed robustness metrics for all Critical Quality Attributes (CQA) studied, into method development experiments. This novel methodology automates a best-practices approach in which HPLC methods can be rapidly developed and simultaneously optimized for mean chromatographic performance and robustness.
|
|
|
|
Pharma Customer Benchmarking
|
|
|
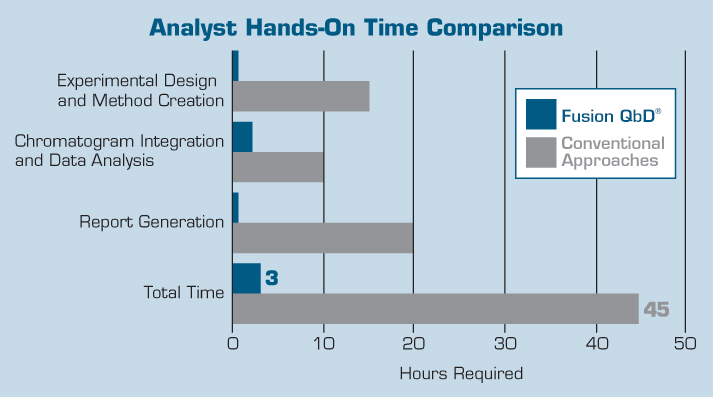
Recent work conducted at a large pharmaceutical company to benchmark the effectiveness of FMD used in combination with Empower 2 and ACQUITY UPLC demonstrated that it was possible to reduce method development time for a complex drug product from 45-60 days to two days when compared to the conventional one-factor-at-a-time (OFAT) development approach.
|
|
|
|
|
|
|
|
Many Fusion users have confirmed that FMD has enabled them to identify truly optimized, robust methods which they never would have been able to discover using their conventional approaches and existing method development software.
“Using Fusion for LC method development is like putting on glasses you never knew you needed” Dan Prudhomme, Research Scientist, Gilead Sciences, Foster City, CA
“After a single set of overnight HPLC runs, Fusion identified the appropriate column and conditions necessary for separating a multi-component mixture containing a pharmaceutical product from three known synthetic intermediates, four known related impurities and revealed four new related impurity peaks, something a contract method development laboratory had been unable to do over several months and at great cost”. Dr Tim Eckersley, Cambridge Isotope Laboratories, Andover, MA
For more information on Fusion LC Method Development, contact us at 707-441-0404 or sales@smatrix.com
|
|
|
|
Application Notes
|
|
|
Fusion LC Method Development has been in use for a number of years and customers have successfully applied FMD to develop and optimize LC methods according to QbD guidelines for a wide variety of sample types, including small molecules, peptides, proteins, and nucleotides. Fusion AE supports a wide range of chromatographic techniques for these samples, including reversed phase, normal phase, ion exchange, HILIC and Chiral separations, and it has never failed to identify an improved method which meets performance requirements.
Below are the first of many application notes we will be posting here in the coming months which demonstrate the power and efficiency of Fusion AE for chromatographic methods development.
|
|
|
|
|
| View |
Download
(PDF, 207 KB)
|
Application Note 001-09
Rapid development of an LC method for separating high molecular weight degradants from a biopharmaceutical product using an automated Design of Experiments (DOE) approach.
|
| View |
Download
(PDF, 723 KB)
|
Application Note 002-09
Using a Design of Experiments Approach to Develop Fast LC Methods for Automated Scale-up to Preparative Chromatography of Sulfa Drugs
|
|
|
|
|
Technical White Papers
|
|
|
|
|
|
|
Download
(PDF, 857 KB)
|
Part 1 of 3
A Quality-by-Design Methodology for Rapid HPLC Column and Solvent Selection
|
Download
(PDF, 1.03 MB)
|
Part 2 of 3
A Quality-by-Design Approach to Rapid Development of Robust HPLC Methods
|
Download
(PDF, 962 KB)
|
Part 3 of 3
Automating HPLC Analytical Method Development
Fusion AE™ Software Program White Paper
|
|

