Fusion Process Development (FPD) is a general Design of Experiments (DOE) module which plugs into the Fusion QbD platform in the same fashion as the Fusion LC Method Development (FMD) and Fusion LC Method Validation (FMV) modules. It connects into the same Fusion Administrator controlled 21 CFR 11 Regulatory Compliance and Workflow Management System feature sets, and has the same internal e-signature and audit trail features.
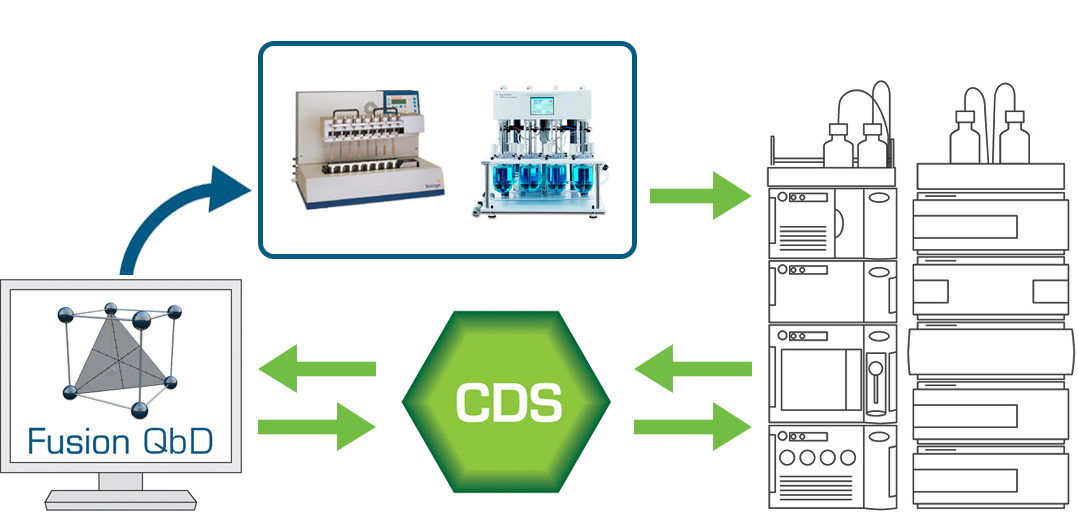
The experiment setup interface is generalized, so the user can create any kind of experiment for any kind and combination of user-defined variables. FPD can also create testing designs for the experiment design which can be exported to your Chromatography Data Software (CDS – connectivity includes Agilent ChemStation, Thermo Chromeleon, and Waters Empower) when some of the results testing is to be done on LC or GC, and of course automatically capture the chromatographic results and remap them to the experiment design for data analysis, response graphing, robustness analysis, numerical and graphical optimization, and its advanced QbD-aligned graphical reporting features.
In the Automated Design mode, FPD automatically selects the most efficient design for you given your phase of the project (Screening or Optimizing) and the variables you have selected for study. In the User-interactive mode you have full control over the choice of design to use and all associated design structure settings. FPD's comprehensive library of statistical experimental design types includes Full and Fractional Factorial, Plackett-Burman, Box-Behnken, Central Composite, Star, Mixed Level, and Model-Robust Algorithm (Letter Optimality) designs for Mixtures, Processes, and Combined (mixture and process).

FPD has the most advanced and complete automated regression modeling capability available today, with integrated error analysis, outlier analysis, transformation analysis, and stepwise regression with built-in diagnostics. In the Automated mode, FPD automatically generates a statistically defensible model of the correct form and order given the experiment design and results data content. In the User-interactive mode you have full control over the choice of starting-point models, the regression analysis approach, and the associated analytic settings.
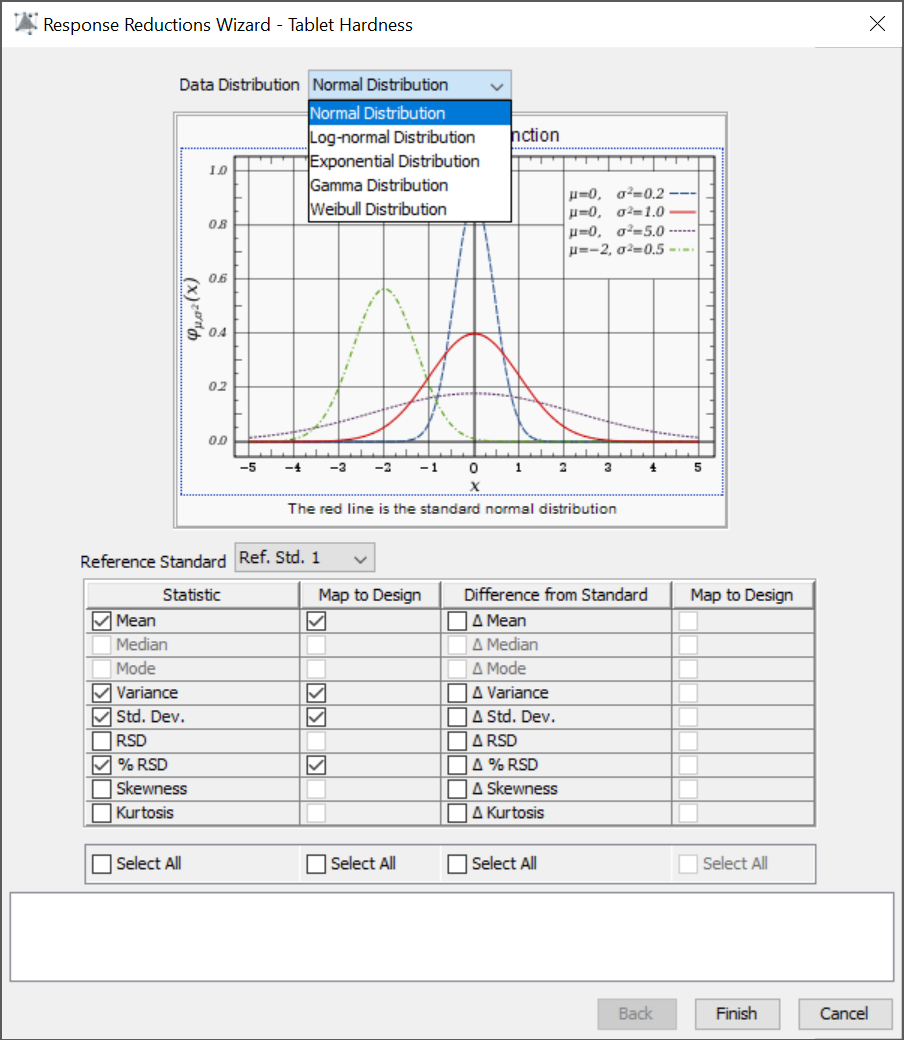
FPD brings a full-featured QbD-based methodology to formulation and process development. Regulatory agencies state that a best practices approach should integrate robustness characterization into R&D studies. Therefore, a critical feature of FPD is the patented Robustness Simulator™ technology (U.S. Patent No. 7,606,685 B2), which can automatically compute process robustness indices such as Cp and CpK for all Critical Quality Attributes (CQA) studied using advanced Monte Carlo simulation techniques. This novel methodology automates a best-practices approach in which formulations and processes can be rapidly developed and simultaneously optimized for mean performance and robustness.
FPD will generate model-based graphics which visualize the relationships between your study variables and your critical performance characteristics (responses). These are displayed as readily interpretable Response Surfaces, contour plots and effects plots ensuring you acquire "process understanding". Instant graphics includes 4-factor trellis graphics — a series of response surface or contour graphs of two graphed variables at user-defined low, medium, and high levels of a 3rd and 4th study variable.
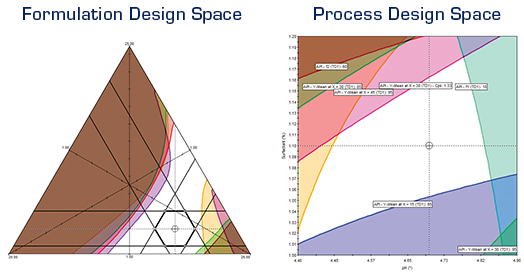
FPD’s powerful optimization tools allow you to search for the conditions that provide the required Critical Quality Attribute goals. Results are produced and displayed in concise tabular and graphical report formats. The Numerical Optimizer reports the search results for each included response (CQA), along with the prediction confidence interval limits, and the "Overall Desirability" of the results relative to your defined performance goals. The Graphical Optimizer translates the modeled experiment results into a visual display of the overlapping study variable conditions in which all response (CQA) quality and performance goals are simultaneously met.


